INTERNATIONAL INVESTMENT
AND PORTAL
Abbott, a global healthcare company, received authorisation from the Department of Medical Equipment and Construction under the Ministry of Health for the sale of its Panbio™ COVID-19 Antigen Self-Test for use in the detection of the SARS-CoV-2 virus.
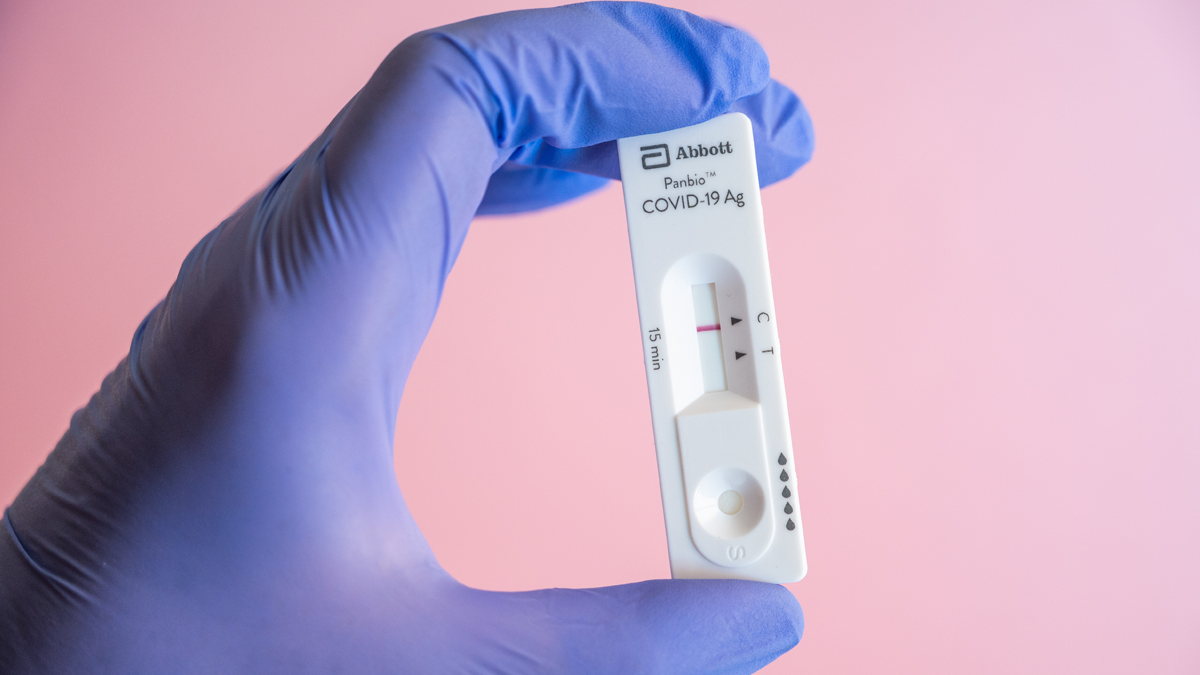 The Ministry of Health greenlit Abbott’s Panbio™ COVID-19 Antigen Self-Test
The Ministry of Health greenlit Abbott’s Panbio™ COVID-19 Antigen Self-Test
With this approval, Abbott brings a non-prescription, rapid antigen nasal self-test to Vietnam for use with or without symptoms.
Frequent rapid testing at work, school, home, and other facilities makes it possible for people to know their infection status when it matters most – making frequent screening an essential tool in the mitigation of COVID-19. According to a recent study published at JAMA, at least 50 per cent of COVID-19 infections are estimated to have originated from exposure to asymptomatic individuals.
"To protect public health, we need to remain vigilant in identifying and slowing the spread of the virus through frequent testing, even with vaccinations," said Sanjeev Johar, divisional vice president for Abbott's rapid diagnostics business in the Asia-Pacific region.
"With authorisation for the Panbio Self-Test, we are able to get these rapid tests into the communities that need them to enable testing at home, school, and work to identify active infections."
Frequent testing makes it possible for people to know their infection status when it matters most. Self-testing has been authorised throughout the world and is widely used throughout North America, Europe, and Asia.
The Panbio self-test is a fast and easy-to-use test that can be used on symptomatic or asymptomatic adults and children, including infants with an adult’s support.
People need to perform a minimally invasive nasal swab and follow a few additional steps. Children under 14 should be supervised by an adult. The test provides results in 15 minutes, no instrumentation is required. Available at pharmacies nationwide, all materials required to perform the test, such as instructions, swabs, test devices, and reagents, are provided in the box.
The Panbio COVID-19 Antigen is one of the most widely studied and used rapid antigen tests in the world. In clinical evaluations with self-test users, the test correctly identified 95.2 per cent of positive samples and 100 per cent of negative samples. All samples were confirmed positive or negative by the Panbio COVID-19 Antigen Rapid Test Device (Nasopharyngeal).
 The Panbio™ COVID-19 Antigen Self-Test is approved for over-the-counter sales for adults and children with or without symptoms, enabling fast and convenient coronavirus testing
The Panbio™ COVID-19 Antigen Self-Test is approved for over-the-counter sales for adults and children with or without symptoms, enabling fast and convenient coronavirus testing
Diagnostics have been a focal point of the COVID-19 response, and Abbott’s main goal has been to get as many tests as possible to as many people as possible.
To date, Abbott has introduced 12 different COVID-19 tests to work in a variety of settings and stages of infection, including the fastest molecular point of care platform, lab-based molecular and serology tests, professional rapid antigen tests, and self-tests. To date, Abbott shipped more than a billion tests worldwide to meet testing needs.
In addition to the Panbio COVID-19 Self-Test, Abbott also offers the Panbio COVID-19 Antigen Rapid Test Device for professional use which is CE-Marked and has received the World Health Organization's approval for emergency use.
By Ngan Vu